What is Lithium Hydroxide and where do we use it?
| Jerry Huang
Lithium hydroxide is a strong base, and its chemical properties are more similar to the Group 2 hydroxides in the periodic table, instead of the Group 1 hydroxides. Lithium hydroxide, chemical formula as LiOH, molecular weight 23.95, white tetragonal crystal, is intensely corrosive and irritating to human skin, with its melting point 450℃ (842℉), relative density as 1.46. Its differentiation temperature is 924℃ (1695℉). It is slightly soluble in ethanol, soluble in water, but its solubility is lower than that of hydroxides of other alkali metals.
After absorbing moisture in the air or crystallizing in an aqueous solution, the lithium hydroxide monohydrate is obtained. Lithium hydroxide reacts with acid gases such as sulfur dioxide, hydrogen chloride, hydrogen cyanide, etc. It can also fully react with strong or weak acids in aqueous solutions. It absorbs carbon dioxide in the air to produce lithium carbonate. Lithium hydroxide is used as a grease additive (thickener, antioxidant, extreme pressure agent), which can improve heat resistance, water resistance, stability and mechanical properties; and lithium-based grease is often used for bearings in automobiles, trains, airplanes, cranes and any precious machines.
Lithium hydroxide battery grade, with low melting point, has been prevailingly accepted as a better electrolyte material in NCA, NCM lithium-ion battery manufacture, which enables nickel-rich lithium batteries much better electric properties than lithium carbonate; while the latter remains the priority choice for LFP and many other batteries so far.
The roasted solid lithium hydroxide can be used as a carbon dioxide absorbent for crews in spacecraft and submarines. Carbon dioxide can be easily absorbed in gas containing water vapor. The chemical reaction formula is as follow: 2LiOH+CO2→Li2CO3+H2O
One gram of anhydrous lithium hydroxide can absorb 450ml of carbon dioxide, and 750g is enough to absorb all the exhaled carbon dioxide from one person in a whole day. In addition, lithium hydroxide is mainly used to produce different lithium compounds and lithium salts, as well as lithium soaps, lithium-based greases and alkyd resins. And it is widely used as catalysts, photographic developers, developing agents for spectral analysis, additives in alkaline batteries. As an additive for alkaline battery electrolyte, lithium hydroxide can increase the electric capacity by 12% to 15% and battery life by 2 or 3 times.
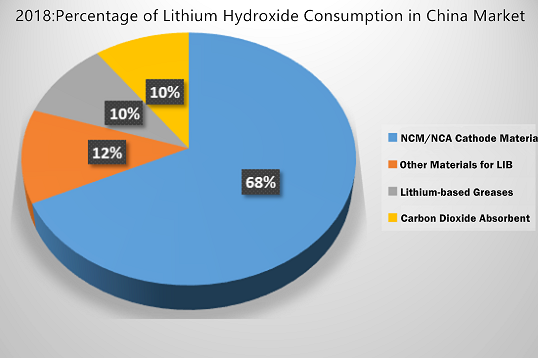
Here is a picture of lithium hydroxide consumption in China market in 2018, for your reference.